This page is part of the 臺灣重大傷病實作指引 (v1.0.1: STU1.0.1) based on FHIR (HL7® FHIR® Standard) R4. This is the current published version. For a full list of available versions, see the Directory of published versions
Profile: 重大傷病申請書回覆-QuestionnaireResponse TWCI
| LinkID | Text | Definition | Answer |
|---|---|---|---|
 | Questionnaire:Catastrophic-Illness-Form | ||
  | hosp|院所資訊 | ||
   | hosp.applMode|申報方式 | NHI-健保重大傷病-申報方式 2: 院所代辦 | |
   | hosp.applType|申報類別 | NHI-健保重大傷病-申報類別 1: 送核 | |
   | hosp.applDate|申請日期 | 2024-01-01 | |
   | hosp.medCertBookDate|開立診斷書申請日期 | 2025-10-20 | |
   | hosp.hospId|醫事機構代碼 | NHI-健保重大傷病-特約醫事機構 0131060029: 衛生福利部臺北醫院 | |
   | hosp.acptNo|受理編號 | 11218899999 | |
  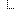 | hosp.acptNum|受理次數 | 1 | |
  | patient|病人資訊 | Patient/pat-min | |
  | doctor|醫師資訊 | ||
   | doctor.diagPrsnId|醫師身分證號 | A234649456 | |
  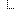 | doctor.diagPrsnName|診斷醫師姓名 | 王小明 | |
  | diagnosis|疾病資訊 | ||
   | diagnosis.icd10cmCode|主診斷代碼 | 臺灣健保署2023年中文版ICD-10-CM C49.6: 軀幹結締及軟組織之惡性腫瘤 | |
   | diagnosis.examinationReport|檢查報告 | ||
    | diagnosis.examinationReport.reportType|報告類型。當LOINC無法具體描述檢體種類(例如:`47526-9`時),請填寫及補充說明檢體種類。 | LOINC 66117-3: Prostate Pathology biopsy report | |
    | diagnosis.examinationReport.speType|檢體種類 | Prostate ; Stomach | |
    | diagnosis.examinationReport.reportResultString|報告結果-文數字 | Prostate labeled as lesion 1 magnetic resonance-ultrasound fusion biopsy adenocarcinoma Gleason score 3+3=6Prostate labeled as lesion 2 magnetic resonance-ultrasound fusion biopsy adenocarcinoma Gleason score 3+3=6Prostate right lateral magnetic resonance-ultrasound fusion biopsy prostatic intraepithelial neoplasia high-gradeProstate right medial magnetic resonance-ultrasound fusion biopsy adenocarcinoma Gleason score 3+3=6Prostate left medial magnetic resonance-ultrasound fusion biopsy adenocarcinoma Gleason score 3+3=6Prostate left lateral magnetic resonance-ultrasound fusion biopsy adenocarcinoma Gleason score 3+4=7MACROSCOPYSites of biopsy: A. Lesion 1 (left anterior midgland TZ): 3 cores (length: up to 2.1 cm) B. Lesion 2 (right anterior apex TZ): 3 cores (length: up to 2.0 cm) C. Right lateral: 3 cores (length: up to 1.8 cm) D. Right medial: 3 cores (length: up to 1.5 cm) E. Left medial: 3 cores (length: up to 1.4 cm) F. Left lateral: 3 cores (length: up to 1.2 cm)All for section and labeled as: A1 to F1. Jar 0MICROSCOPY1. Histologic diagnosis: A. Lesion 1: adenocarcinoma * Gleason score: 3+3=6 (Grade group: 1) * Area percentages of tumor part: 90% 70% 60% * Maximum cancer core length: 9.8 mm B. Lesion 2: adenocarcinoma * Gleason score: 3+3=6 (Grade group: 1) * Area percentages of tumor part: 40% 25% 5% C. Right lateral: high-grade prostatic intraepithelial neoplasia (PIN unifocal) D. Right medial: adenocarcinoma * Gleason score: 3+3=6 (Grade group: 1) * Area percentages of tumor part: 20% 0% 0% E. Left medial: adenocarcinoma * Gleason score: 3+3=6 (Grade group: 1) * Area percentages of tumor part: 40% 40% 0% F. Left lateral: adenocarcinoma * Gleason score: 3+4=7 (Grade group: 2) * Percentage of pattern 4 component: less than 5% * Area percentages of tumor part: 100% 5% 5%2. Perineural invasion: not identified3. Extraprostatic extension: not identified4. Seminal vesicle invasion: seminal vesicle not includedStomach middle to upper body lesser curvature anterior wall endoscopic biopsy (1) extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) (2) chronic active gastritis with Helicobacter infection (3) intestinal metaplasiaStomach antrum lesser curvature and greater curvature endoscopic biopsy (1) chronic gastritis without Helicobacter infection (2) intestinal metaplasiaStomach mid-body lesser curvature and greater curvature endoscopic biopsy (1) chronic gastritis with Helicobacter infection (2) intestinal metaplasia The specimen is submitted in 3 separated bottles labeled as A B and C respectively fixed in formalin. The specimen A consists of two tissue fragments measuring up to 0.4 x 0.2 x 0.2 cm in size. Grossly they are gray-white and soft. The specimen B consists of two tissue fragments measuring up to 0.3 x 0.2 x 0.2 cm in size. Grossly they are gray-white and soft. The specimen C consists of 3 tissue fragments measuring up to 0.3 x 0.2 x 0.2 cm in size. Grossly they are gray-white and soft. All for section and labeled as. | |
    | diagnosis.examinationReport.reportResultPdf|檢查報告檔案,請填寫完整檔案路徑。填寫格式:「file://檔名.副檔名」。 | file://PathologyReport01.pdf | |
    | diagnosis.examinationReport.reportResultPdfTitle|檢查報告名稱 | PathologyReport01 | |
   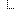 | diagnosis.examinationReport.reportDate|報告日期,YYYY-MM-DD。 | 2024-01-01 | |
   | diagnosis.medrec|病歷資料 | ||
    | diagnosis.medrec.medrec|病歷資料 | file://Medicalrecord01.pdf | |
   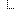 | diagnosis.medrec.medrecTitle|病歷資料名稱 | Medicalrecord01 | |
   | diagnosis.imageStudy|影像報告 | ||
    | diagnosis.imageStudy.imgItem|影像報告 | 臺灣健保署2023年中文版ICD-10-PCS B34JZZ3: 左上肢動脈血管內超音波 | |
    | diagnosis.imageStudy.imgResult|影像報告結果 | CT of Chest with contrast enhancement shows: COMPARISON: 2023-12-13. FINDINGS: - placement of a right port-A catheter. - a small(<=6mm) perifissural nodule in right minor lung fissure(SE2 IM66) larger. - small pleural nodules at RUL and LUL up to 1.1cm in apical RUL larger. - no pleural effusion. - no definite mediastinal lymphadenopathy. - some mixed increased and decreased densities at the vertebral bodies of thoracolumbar spines; partial collapse of L5 vertebral body stable. 1. A small perifissural nodule in right minor lung fissure larger. 2. Small pleural nodules at RUL and LUL up to 1.1cm in apical RUL larger. 2. Bone metastases. | |
    | diagnosis.imageStudy.imgDate|影像報告日期 | 2024-01-01 | |
    | diagnosis.imageStudy.imgBodySite|影像檢查的身體部位 | SNOMED CT 774007: Head and neck structure | |
    | diagnosis.imageStudy.imgDicom|DICOM影像 | ||
     | diagnosis.imageStudy.imgDicom.studyUid|整項影像檢查的識別碼 | urn:oid:2.16.886.2102.54.4546465747.465465465 | |
     | diagnosis.imageStudy.imgDicom.series|每項影像檢查有一個或多個系列(series)的實例 | ||
      | diagnosis.imageStudy.imgDicom.series.uid|此系列的DICOM系列實例UID | 2.16.886.2102.54.4546465747.465465466 | |
      | diagnosis.imageStudy.imgDicom.series.modality|此系列實例所使用的成像儀器 | DICOM CT: Computed Tomography | |
      | diagnosis.imageStudy.imgDicom.series.instance|系列中的一個SOP實例 | ||
       | diagnosis.imageStudy.imgDicom.series.instance.uid|DICOM影像 | 2.25.88017001449189502323411118737039844241 | |
       | diagnosis.imageStudy.imgDicom.series.instance.sopClass|DICOM class 類型 | unknown urn:oid:1.2.840.10008.5.1.4.1.1.2: urn:oid:1.2.840.10008.5.1.4.1.1.2 | |
    | diagnosis.imageStudy.imgNonDicom|非DICOM影像 | ||
     | diagnosis.imageStudy.imgNonDicom.imgNonDicom|非DICOM影像 | file://US01.jpg | |
     | diagnosis.imageStudy.imgNonDicom.imgNonDicomMimeType|非DICOM影像MimeType | urn:ietf:bcp:13#image/jpeg | |
  | ci|重大傷病 | Condition/con-min | |
  | cancerStage|癌症期別 | ||
   | cancerStage.cancerStage|癌症期別,醫院自行填入癌症期別(1~4),若為不適用者填9(不適用)。 | NHI-健保重大傷病-癌症期別 1: 第一期 | |
   | cancerStage.assessScore|癌症分期分數或結果 | T1 | |
   | cancerStage.assessDate|癌症分期量表評估日期,YYYY-MM-DD,西元年月日,民國前為負數。 | 2024-01-01 | |
  | illness|惡性腫瘤重大傷病換發評估表 | ||
   | illness.oriCancerCode|原發癌症診斷碼,最長為7碼。 | 臺灣健保署2023年中文版ICD-10-CM C49.4: 腹(部)結締及軟組織之惡性腫瘤 | |
   | illness.oriCancerDxDate|癌症最初診斷日期,西元年月日;不得大於系統日。 | 2017-03-16 | |
   | illness.oriCancerAjcc|癌症最初診斷AJCC分期(病理分期或未接受治療前的臨床分期),依期別填入;若不是用此分類而用其他分類,則填寫9。 | NHI-健保重大傷病-癌症最初診斷AJCC分期 9: 其他系統 | |
   | illness.oriCancerAjcc1|癌症最初診斷AJCC分期_補充說明欄位,若前述欄位為9,則請於此欄位描述其他系統之其他分期為何。 | T1 | |
   | illness.cancerStatus|目前癌症狀態 | NHI-健保重大傷病-癌症狀態 4: 癌症遠端轉移 | |
   | illness.cancerTreatment|後續治療評估,可複選。 | NHI-健保重大傷病-後續治療評估 1: 需定期返診追蹤檢查 NHI-健保重大傷病-後續治療評估 5: 癌症後遺症及併發症治療 | |
   | illness.cancerTreatmentPlan|後續治療計劃,可複選。 | NHI-健保重大傷病-後續治療計劃 1: 手術治療 NHI-健保重大傷病-後續治療計劃 2: 放射線治療 | |
   | illness.cancerTreatmentText|補充說明。 | 補充說明 | |